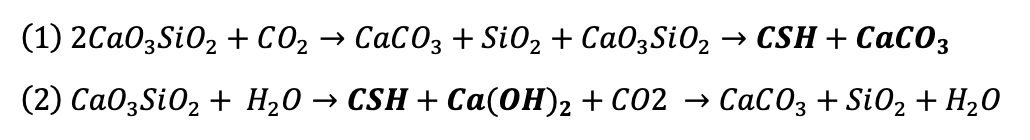Carbon dioxide curing and natural carbonation are often mistakenly seen as the same process. While they share a common chemical foundation, they differ significantly in timing, mechanism, and impact. Understanding these differences is essential for researchers, scientists, and technology leaders working with concrete and carbon dioxide utilisation.
Carbon dioxide curing is not accelerated natural carbonation.
Both carbon dioxide curing and natural carbonation involve a reaction between carbon dioxide and alkaline materials in concrete, primarily calcium. However, the pathways diverge.

Same Chemical Basis But Different Timescale and Outcome
In carbon dioxide curing, CO₂ reacts directly with dissolving cement during the early stages of hardening. In contrast, natural carbonation occurs later, as CO₂ interacts with cement hydration products over time in surfaces of hardened concrete structure.
This distinction matters. When carbonates form directly during curing, they densify the microstructure of the hardening concrete. Additionally, the silica (SiO₂) released in the process can react with unhydrated cement, further enhancing the material’s performance.
Carbon dioxide curing and natural carbonation share a chemical basis, but differ in timing, mechanism, and outcome.
Equations 1 and 2 present the carbon curing process and natural carbonation, respectively. The composition of hardened concrete is presented in bold in the equations.
Natural carbonation is a slow process that begins at the surface and progresses inward after the concrete has hardened. Carbon dioxide curing, on the other hand, takes place during the hardening phase itself.
This timing is critical. If CO₂ is introduced after hardening, the process becomes merely accelerated natural carbonation. But when applied during hardening, carbon dioxide curing produces a combination of calcium silicate hydrate (C-S-H) and calcium carbonate (CaCO₃). This preserves the concrete’s essential properties, such as high pH and a dense microstructure.
Natural carbonation continues throughout the concrete’s lifetime. Due to dense microstructure, significant CO₂ uptake only occurs if the concrete is crushed and exposed to CO₂ at the end of its life.
In contrast, carbon dioxide curing stores CO₂ permanently during the manufacturing phase, contributing to cradle-to-gate decarbonisation.
Does Carbon Dioxide Curing Affect Natural Carbonation?
Experimental studies show that carbon dioxide curing does not alter the rate of natural carbonation. This is because natural carbonation depends on the transport properties of the concrete – its microstructure – rather than its chemical history.
For customers, this means there is no increased risk of premature reinforcement corrosion due to carbon dioxide curing. It also confirms that the process does not significantly reduce the CO₂ uptake from natural carbonation over the concrete’s lifetime. The only impact is on the end-of-life accelerated carbonation, which becomes less relevant when CO₂ is already stored during production.
Carbon dioxide curing is a beneficial process due to its effect of densifying the concrete microstucture

About Carbonaide
Cheaper, faster, stronger and greener. Our mission is to turn building materials from a large emission source into a carbon sink. With the Carbonaide CO2 curing solutions, concrete manufacturers can utilise carbon dioxide to improve their production and permanently store carbon as carbonates in the products at the same time.